Despite multiple lawsuits in Canada and the United States, and warnings from Health Canada and the FDA, reports of complications from Mirena, a popular method of birth control, have increased by 81 per cent in Canada since 2009.
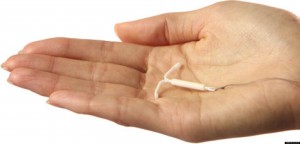
Mirena is an intrauterine contraceptive device (IUD) created by the pharmaceutical company Bayer. It’s a T-shaped birth control device that is inserted in a woman’s uterus with the intention to prevent pregnancy for up to five years. It was approved by the FDA in 2000 and by Health Canada a year later.
In 2009 there were 93 reports filed with Health Canada of adverse effects from the Mirena IUD. That number grew to 500 reports in 2013 alone. In the last five years almost 1600 reports of complications that were considered serious have been reported.
Since Mirena was approved, class action lawsuits against the IUD have been popping up across Canada and the United States. In Nova Scotia, Wagners law firm launched a case against Bayer in 2013.
Mike Dull, a lawyer on the case, says 30 women have already contacted them. All thirty women have had the Mirena IUD perforate their uterus or migrate to other parts of their body. Some have lost their ability to have children through the damage done by perforations or migrations, while Amy Tudor, the representative for the class, ended up with an unexpected pregnancy.
“They’re upset,” says Dull. “These ladies decided to put this particular product inside them and the ones that I’ve spoken with have had very serious consequences.”
On their website Bayer describes Mirena as “highly effective”, “convenient” and “reversible”. At the bottom of the page they warn, “Mirena may attach to or go through the wall of the uterus and cause other problems.”
The “other problems” that can be caused by perforation or migration range from pelvic inflammatory disease, to the risk of ectopic or intrauterine pregnancy.
In their product monograph, a written account of studies done on a product, Bayer says the chances of uterine perforation are between 1 in 1,000 and 1 in 10,000.
Last year alone Health Canada received 55 reports of uterine perforation caused by Mirena. Since 2009, there have been 239 reports of perforations from the IUD.
“The numbers are extremely low,” says Dull. “Those are people who took the active step in going and reporting to Health Canada.”
Kathleen, who asked to only be identified by her first name due to the personal nature of her experience, had her first Mirena IUD inserted six years ago and had complications. “The only complications I was warned about was prolonged spotting,” she says, “and it possibly falling out.”
Eight months after the insertion Kathleen says she had severe cramping and discovered the IUD had embedded itself into her uterine wall. She had to have it surgically removed. Five months after the surgery she had a second IUD inserted.
Nine months after the second insertion Kathleen found out she was pregnant. She says due to the scaring from the first Mirena she had an ectopic pregnancy which resulted in termination of the pregnancy. “The damages were assessed and I was told I would never conceive a child naturally,” she says.
Unexpectedly in 2013 Kathleen discovered she was pregnant and was sent to a high risk obstetrician. She had a complicated pregnancy due to the damage done by Mirena but fortunately gave birth to a healthy baby girl who is now six months old.
Dull says a small number of the 30 women who have joined the class action now have fertility problems due to Mirena.
“There are women who cannot have children because of this product,” says Dull. “That’s not what they intended of course when they put in a temporary birth control mechanism. They’re often younger girls as well and they will forever be suffering the consequences of that.”
According to Dull there are about four firms in Canada working on a class action against Bayer for the Mirena IUD. Wagners is working with two of them to potentially bring the class action to Alberta where they have an established case law for class actions and the court system is quicker.
In 2010 Health Canada, the FDA and Bayer all released safety information regarding Mirena. It was to clarify the potential risks of uterus perforation and migration of the IUD that were not made clear by Bayer previously.
Even though safety information has been released and updated since Mirena was approved in 2001 and lawsuits keep piling up, the number of adverse effects from Mirena continue to grow. In the first three months of 2014 there were already 18 reports of uterine perforation which makes up 20 per cent of the complaints.
Dull says he’s heard from some doctors who say Mirena is not fit for its intended use as a birth control method. “There’s other products out there that do the exact same thing,” says Dull, “and don’t carry with it the same risks.”
The Halifax Sexual Health Centre was contacted but they said they do not sell Mirena because it is too expensive for their patients so they would not be able to comment.
Going forward with the class action Dull believes that Bayer, even though he says they are denying all allegations, will defend the case for as long as possible and put off any sort of settlement, that way they can continue to make money off their product.
Dull also believes that Health Canada is partially to blame. “Health Canada in its regulatory regime, is intended of course to protect Canadians from devices like this and bad drugs, like the FDA does in America,” says Dull, “They’re meant to do it, but I don’t know the last time they actually imposed anything on a medical device company.”
He hopes that with this class action will come change. He would like to see Health Canada investigate, hire experts and do a study to determine if Mirena is a product Canadians should be using.
