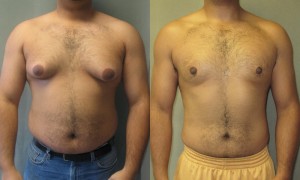
Two Canadian law firms are teaming up and combining their class actions against Janssen inc. over an antipsychotic medication that caused gynecomastia (male breasts) in patients.
The drug, Risperdal was not approved for treating minors. It is a potent antipsychotic, but has been prescribed for treating ADHD in youths.
The product label only mentions gynecomastia as a side-effect for adults, but does suggest that metabolic side-effects were more prevalent in minors during testing.
To put it simply, Toronto lawyer Bryan McPhadden says, “We allege that they (the patients) were not warned correctly about the risks of gynecomastia.”
McPhadden’s firm is teaming up with Joseph Prodor’s Vancouver based firm in what now appears to be a unified class action against Janssen inc., a branch of Johnson and Johnson.
As recently as July, 24 McPHadden had been pursuing class actions separate from Prodor’s in a number of Canadian Provinces, most recently in Nova Scotia where a class action was launched on behalf Ryan Hanna, an 18 year of man from Sydney NS that had breast reduction surgery to reduce the breasts he developed after taking the drug for nearly a decade. The legal filings allege that the breasts were a direct result of years of prescribed Janssen inc. ADHD medications: Risperdal and Invega.
The drugs have a long legal history that has resulted in class actions across the globe, most notably in The United States where both citizens and States have sought and received financial compensation on behalf of boys who developed breasts as a result of taking the drug.
These lawsuits have centred around two key arguments: 1) the product labels did not indicate the risks associated with the drug and 2) the pharmaceutical representatives that sold and promoted the drugs acted unlawfully in their promotion of the drug for off-label purposes.
In Canada it’s a bit of a different case. Risperdal was never approved for treating minors; though in Canada doctors are allowed to prescribe any medication that they feel will help a patient. The Canadian cases our based upon the notion that patients were not warned of the risks of developing gynecomastia.

Court documents show that weight gain was a known side effect of the drugs, but the plaintiffs argue that weight gain actually made it more difficult to detect the enlarged breasts.
For over a decade Philadelphia attorney Steve Sheller has brought and won several actions over the same drug. The cases are at various stages, but some have resulted in multi-million dollar payouts to the plaintiffs. The total paid out thus far in the US has been over an estimated 1.6 Billion dollars.
Sheller’s cases were strengthened by proving that J&J engaged in off-label promotional activities such as financial rewards for prescribing the drugs.
Sheller’s class actions took in many cases over five years to resolve and many remain in courts yet unsettled.
It remains to be seen how the cases in Canada will play out, but the McPhadden and Prodor’s consortium is encouraging to the plaintiffs’ cause.
The medical side

Dr. Steve is an American physician who did not want to be fully identified due to the controversial nature of this case, he sympathizes with the plaintiffs, but also sees the ambiguous proof that the judgments rely on.
“Teenagers and young adults are very body-conscious and gynecomastia at that age comes at the worst possible time. It can be a huge blow to one’s self-esteem. Although it is correctable, the treatment can be very invasive and carries some risk itself.“
Risperdal can cause an increase in a hormone called Prolactin, this is the cause most lawyers use for gynecomastia (male breasts) in Risperdal users.
However, patients with prolactin secreting tumors rarely get gynecomastia,but high prolactin levels have been blamed for gynecomastia for years.
A recent review of the medical literature said that Risperdal was “probably” associated with gynecomastia. Dr. Steve says “probably” doesn’t by any means mean it does definitively cause gynecomastia.
Some doctors prescribed risperdal despite some early warnings in the literature.
“So as always, it’s not a slam-dunk. The kids who were on this medication were pretty mentally ill to be put on it in the first place.”
Financial Implications
In their 2013 annual financial filings Johnson and Johnson acknowledges the numerous legal actions facing them.
Despite the 1.6-billion USD the company paid out to satisfy Risperdal legal proceedings in the United States in 2013, the company does not foresee ongoing legislation impacting their cash flow going forward.
The company does note that a large one-time payment could have an adverse temporary financial effect in a given quarter or period’s financial statement.
The company has extensive insurance against potentially catastrophic legislation or liabilities against them.
The Canadian and UK class actions against Johnson and Johnson over Risperdal are significantly smaller in the number of plaintiffs in the class and therefore the payouts are expected to be smaller.
Annual sales of Invega were up 6-million USD in 2013, but sales for Risperdal fell 7.5-million USD in 2013 and 17.5-million USD since 2011. The company lists competition from generic drugs as the reason for the decline and not the litigation surrounding the product.
The company’s earnings per share were up nearly 1 USD in 2013 and nearly 1.5 USD since 2011 and the company’s overall stock price increased 33 per-cent in 2013.
Interactive Map: Major Risperdal Related Legal Cases

